Clinical Update Forty-Four: Valve Choice
Valve Choice Has Long-Term Complication and Cost Implications for Patients and Healthcare Providers
Comparative long-term cost model shows choosing the On-X heart valve for implantation in young patients could save: $59,000 over choosing a stented tissue (ST) valve with ST at re-op1, $376,000 over choosing a stented tissue (ST) valve with TAVI/ ViV at re-op1
Comparative Cost Effectiveness Analysis
In today’s cost-conscious environment, healthcare providers are beginning to compare both the clinical and costeffectiveness of alternative treatment strategies.2 To help with making these important decisions an economic model was developed to determine the life-time complication rates and the costs associated with prosthetic choice.3 This study is the work of Sidney Levitsky, MD of Harvard Medical School and Steven Culler, PhD with Rollins School of Public Health, Emory University.
This model compared the following prosthesis types for the initial surgery and reoperation:
- On-X mechanical valve
- Stented tissue valve replaced with another tissue valve using a surgical aortic valve replacement (SAVR) procedure at reoperation
- Stented tissue valve replaced with a transcatheter valve in valve (ViV) procedure at reoperation
Increased complications results in increased costs
This model examined the patient profile of a typical 55 year old heart valve disease patient requiring an aortic heart valve replacement. Estimated inputs for complication event rates were obtained from peer reviewed clinical journals.1
Clinical event differences between prosthesis types include:
- Thrombotic events
- Bleeding events
- Warfarin and monitoring
- Heart failure onset that precedes structural valve deterioration (SVD) after year 10 (specifically patient decline and adverse clinical events leading to the diagnosis of SVD).
- Reoperation due to structural valve deterioration
- Clinical complications following transcatheter procedures A summary comparing complication results by prosthesis type is presented in Table 1.4
Table 1. Long-Term Event Comparisons: Aortic Valve Replacement (%/pt.yr.)4 (click to enlarge)
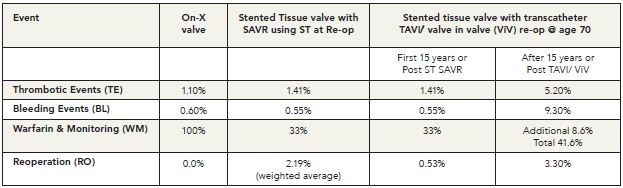
Complications and Cost Implications:
The lifetime costs of heart valve replacement are not inconsequential.2 Table 2 shows the long-term cumulative costs and savings by prosthesis type.5 Lifetime adverse clinical events and costs associated with heart valve replacement include:
- Initial surgery
- Ongoing maintenance: doctor visits, echo monitoring and anticoagulation therapy
- Costs to treat valve related complications: thrombotic events, bleeding events
- Structural valve deterioration (SVD) adverse clinical events and SVD requiring reoperation
The economic model estimates 25-year lifetime cumulative healthcare cost6 to be:
- $124,200 using the On-X Life Technologies mechanical valve (MV) during initial surgery6
- $183,600 using a stented tissue valve (ST) at initial surgery with a second ST with a (SAVR) procedure at reoperation6
- $500,214 using a stented tissue valve (ST) at initial surgery with (TAVI) (ViV) procedure at reoperation6
Implications for Healthcare Policy:
This comparative analysis estimates changing the initial prosthesis choice to the On-X mechanical valve in the estimated 20,000 stented tissue valve surgeries performed annually in the US among patients below age 65 would result in fewer adverse clinical events and a savings of approximately $1.2 billion to $7.5 billion in lifetime direct healthcare expenditures per year.7,8 The majority of savings occur when these patients would likely be Medicare beneficiaries. And the healthcare system could potentially save an estimated $1.2 billion to $7.5 billion reduction in direct healthcare expenditures over the following 25 years depending.
Implications for Patients:
Patients who choose an On-X mechanical valve for their initial heart valve surgery can look forward to a life with fewer adverse clinical events and reduced lifetime costs associated with their heart valve replacement.
References
- Predicting long-term costs of heart valve replacement: A comparative analysis between prosthesis types. On-X Life Technologies, Inc.: Austin, Texas USA 2013. Aggregated peer reviewed references: 1-59 available upon request.
- Caro J, Migliaccio-Walle K, O’Brien JA. The cost of treating heart valve related complications. J Heart Valve Dis 1996;5:122-27
Levitsky S,Culler SD. Predicting long-term costs of heart valve replacement: A comparative analysis between prosthesis types. American College of Cardiology 2013; San Francisco, CA. USA. March 9-13, 13-A-11926-ACC - Predicting long-term costs of heart valve replacement: A comparative analysis between prosthesis types. On-X Life Technologies, Inc.: Austin, Texas USA 2013. Aggregated peer reviewed references: 1-27, 31, 34-40, 44, 46-54, 56 available upon request.
- Predicting long-term costs of heart valve replacement: A comparative analysis between prosthesis types. On-X Life Technologies, Inc.: Austin, Texas USA 2013. Aggregated peer reviewed references: 29-31, 41, 42, 45, 55, 57-59 available upon request.
- Levitsky S, Culler SD. Initial heart valve replacement prosthetic choice has long-term complications and cost impact: A comparative analysis. International Society of Pharmacoeconomics and Outcomes Research (ISPOR) 2013; New Orleans, LA. USA. May 18-22, PCV47
- Wheatley, David J. The ‘Threshold Age’ in choosing biological versus mechanical prostheses in western countries. J Heart Valve Dis 2004;13(Supplement 1):S91-S94
- Health Research International Global Heart Valve Model. Health Research International 2010: Lakewood, OH,USA
On-X aortic and mitral valves are FDA approved. Not all On-X valve models are available in all markets.
CAUTION: Federal law restricts this device to sale by or on the order of a physician. Refer to the Instructions for Use that accompany each valve for indications, contraindications, warnings, precautions and possible complications. For further information, visit www.onxlti.com.
Headquarters and Manufacturing Facilities: 1300 East Anderson Lane, Building B Austin, Texas 78752 U.S.A.
Telephone: (512) 339-8000 – Facsimile: (512) 339-3636 – www.onxlti.com – onx@onxlti.com
010006 223 041713 © 2013 On-X Life Technologies, Inc.



