Clinical Update Forty-Six: Recently Approved in Europe
Recently Approved in Europe — Lower Anticoagulation Maintenance of Aortic Heart Valve Recipients!1
Introducing the On-X® Plus 1.5™ Aortic Heart Valve: Significantly reduced bleeding for On-X aortic heart valve patients does not increase the stroke rate.2
European aortic valve patients can now take less warfarin for lowered bleeding rates
The On-X valve is the first and only mechanical valve to be tested in a randomized, FDA approved clinical trial for lowered levels of INR.2 On-X Life Technologies, Inc. (Austin, Texas) has received European regulatory approval—CE mark (Conformité Européenne mark)—for an aortic valve INR level of 1.5 to 2.0 compared to a standard recommendation of 2.0-3.0.3 Aortic valve patients in countries that recognize the CE mark can now control their anticoagulation level within this lowered labeling indication for On-X Plus 1.5 Aortic Valves.
A mechanical valve that offers less bleeding events
A presentation of the “High Risk” patient group data from the Prospective Randomized On-X Anticoagulation Clinical Trial (PROACT) was made at the American Association of Thoracic Surgery in Minneapolis.2 Bleeding was reduced by 60% and thromboembolism (TE) was not increased for these patients who were testing their INR levels at home (Table 1).
Table 1. PROACT: Adverse Events: High Risk Aortic Test Group (INR 1.5-2.0) vs. Control Group (INR 2.0-3.0)2
| Adverse event (N) | Control | High Risk Aortic | Rate Ratio Test/ Control | P-value |
|---|---|---|---|---|
| Major bleed | 25 | 10 | 0.45 | 0.032 |
| Minor bleed | 25 | 8 | 0.36 | <0.001 |
| Ischemic stroke | 5 | 5 | 1.12 | 0.859 |
| All bleeding and thrombosis | 64 | 38 | 0.66 | 0.046 |
| Total mortality | 11 | 10 | 1.02 | 0.968 |
The stunning result of the PROACT trial is that all bleeding was reduced while stroke and thromboembolic events did not increase. Further, these event rates remain well below FDA’s Objective Performance Criteria (OPC) despite the isolation of high risk patients into this cohort. 4-6
Table 2. Risk Factors: Patients in the PROACT High Risk Aortic Test Group7
| Factors associated with early degeneration and reoperation of tissue valves |
| Chronical atrial fibrillation |
| Left ventricular ejection fraction <30% |
| Enlarged left atrium >50mm diameter |
| Spontaneous echo contrasts in the left atrium |
| Vascular pathology |
| Neurological events |
| Hypercoagulability |
| Left or right ventricular aneurysm |
| Lack of platelet response to aspirin or clopidogrel |
| Women receiving estrogen replacement therapy |
The best of both worlds – a long lasting valve and a new, low optimal, range for INR
Prior studies showing the relationship of bleeding, thromboembolism and INR have produced curves that provide an INR range between 2.2 and 3 or higher for mechanical valve use.8-10 The High Risk Aortic Test Group of the PROACT trial has provided evidence for a dramatic, reduced alteration and lowering of the INR optimal range as seen in Figure 1.2
Figure 1. Relationship of Bleeding to Thromboembolism2
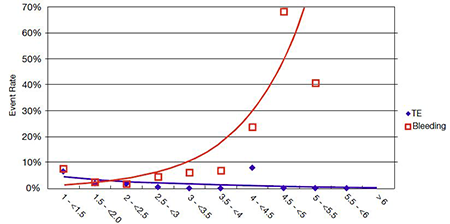
Studies show that patients who are 65 or younger should receive mechanical valve implants to avoid the complications associated with repeated reoperations.11,12 Mechanical valve patients have better long term survival at any age.11-15 The benefits of warfarin in older patients 16-18 have been overlooked in favor of patient preference for tissue valves that is based on somewhat unfounded fears. While uncontrolled warfarin use can be dangerous especially with respect to bleeding, patient self-monitoring has been shown to cut bleeding complications.19-21
The On-X valve provides the advantage of low reoperation rates, low mortality and reduced complications22,23 — the best of all worlds for a young valve recipient.
Your patients deserve the best chance for a long lasting valve without high bleeding rates — the On-X Plus 1.5™ Aortic Heart Valve!
References
- Ford O. On-X gains CE mark for expanded indication of prosthetic heart valve. Medical Device Daily 2014;18(13):1,5
- Puskas JD. On behalf of the PROACT Investigators. Reduced anticoagulation after mechanical valve replacement: Interim results from the PROACT randomized FDA IDE trial. Presented May 6, 2013 at the American Association of Thoracic Surgery Annual Meeting 2013 in Minneapolis Minnesota;http://webcast.aats.org/2013/files/Monday/20130506_auditorium_0745_07.45%20John%20D.%20Puskas.mp4
- ACC/AHA 2006 Guidelines for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the1998 Guidelines for the Management of Patients With Valvular Heart Disease): Developed in Collaboration With the Society of Cardiovascular Anesthesiologists Endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons Circulation 2006;114;84-231. DOI: 10.1161/CIRCULATIONAHA.106.176857
- Food and Drug Administration; Draft Guidance for Industry and FDA Staff: Heart Valves – Investigational Device Exemption (IDE) and Premarket Approval Applications, January 10, 2010
- ISO 5840, Standard for Cardiovascular Implants – Cardiac Valve Prostheses; 2005
- On-X® Prosthetic Heart Valve. Summary of Safety and Effectiveness Data submitted to the United States Food and Drug Administration. PMA P000037. Approval date May 30, 2001 and March 6, 2002
- PROACT Investigation Plan. Medical Carbon Research Institute, LLC, Austin, Texas USA; © 2006
- Horstkotte D, Schulte H, Bircks W, Strauer B. Unexpected findings concerning thromboembolic complications and anticoagulation after complete 10 year follow-up of patients with St. Jude Medical prostheses. J Heart Valve Dis 1993;2:291-301
- Bodnar E, Horstkotte D. Potential flaws in the assessment of minor cerebrovascular events after heart valve replacement. J Heart Valve Dis 1993;2:287-90Philips SJ. Searching for the truth: a mechanical or a tissue valve? J Heart Valve Dis 2004;13(Suppl.1):S95-S98
- Cannegieter SC, Rosendaal FR, Wintzen AR, et al. Optimum oral anticoagulant therapy in patients with mechanical heart valves. N Engl J Med 1995;333:11-17
- Brown ML, Schaff HV, Lahr BD, et al. Aortic valve replacement in patients aged 50 to 70 years: Improved outcome with mechanical versus biologic prostheses. J Thorac Cardiovasc Surg 2008;135:878-84
- Weber A, Noureddine H, Englberger L, et al. Ten-year comparison of pericardial tissue valves versus mechanical prostheses for aortic valve replacement in patients younger than 60 years of age. J Thorac Cardiovasc Surg. 2012;144(5):1075-83
- Stassano P, Di Tommaso L, Monaco M, et al. A prospective randomized evaluation of mechanical versus biological valves in patients ages 55 to 70 years. JACC 2009;54:1862-68
- Vicchio M, Della Corte A, De Santo LS, et al. Tissue versus mechanical prostheses: Quality of life in octogenarians. Ann Thorac Surg 2008;85:1290-95
- de Vincentiis C, Kunkl AB, Trimarchi S, et al. Aortic valve replacement in octogenarians: Is biologic valve the unique solution? Ann Thorac Surg 2008;85:1296-1301
- Horton JD, Bushwick BM. Warfarin therapy: Evolving strategies in anticoagulation. Am Family Physician 1999; p. 635
- Smith P, Arnesen H, Holme I. The effect of warfarin on mortality and reinfarction after myocardial infarction. N Engl J Med 1990;323:147-52
- Hurlen M, Smith P, Arnesen H. Effects of warfarin, aspirin and the two combined, on mortality and thromboembolic morbidity after myocardial infarction. Scand Cardiovasc J 2000;34:969-74
- Koertke H, Zitterman A, Minami K, et al. Low-dose international normalized ratio self-management: A promising tool to achieve low complication rates after mechanical heart valve replacement. Ann Thorac Surg 2005;79:1909-14
- Heneghan C et al. Self-monitoring of oral anticoagulation: A systematic review and meta-analysis. Lancet 2006 Feb 4; 367:404-11
- Hirsh JH, Dalen JE, Anderson DR, et al. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest 2001;119:8S-21S
- Williams MA, Van Riet S. The On-X Heart Valve at 10 Years. Presented at the 6th Biennial Meeting of the Society for Heart Valve Disease.June 25-28, 2011 in Barcelona, Spain
- Chambers JB, Pomar JL, Mestres CA, Palatianos GM. Clinical event rates with the On-X bileaflet mechanical valve: A multicenter experience with follow-up to 12 years. J Thorac Cardiovasc Surg 2013;145:420-24 DOI: 10.1016/j.jtcvs.2011.12.059 http://jtcs.ctsnetjournals.org/cgi/content/full/145/2/420
On-X aortic and mitral valves are FDA approved.
The approval of a lower INR recommendation through the EU regulatory process applies only within that jurisdiction and others that accept EU review. This therapy is not approved in the US or other countries that have reviews independent of the EU. In these countries On-X Life Technologies, Inc., continues to recommend standard anticoagulation therapy as presently prescribed by various professional societies for the On-X valve.3
CAUTION: Federal law restricts this device to sale by or on the order of a physician. Refer to the Instructions for Use that accompany each valve for indications, contraindications, warnings, precautions and possible complications. Investigational use of this device in the Prospective Randomized On-X Valve Anticoagulation Trial (PROACT) is limited by federal law to investigational sites.
For further information, visit www.onxlti.com.
Headquarters and Manufacturing Facilities: 1300 East Anderson Lane, Building B Austin, Texas 78752 U.S.A.
Telephone: (512) 339-8000 – Facsimile: (512) 339-3636 – www.onxlti.com – onx@onxlti.com
010006 229 020714 © 2014 On-X Life Technologies, Inc.


