Clinical Update Forty-Two: Mitral Valve
Optimal flow in a mitral valve
Less mismatch with the On-X mitral valve proves that optimal flow is achieved at a smaller nominal size.1
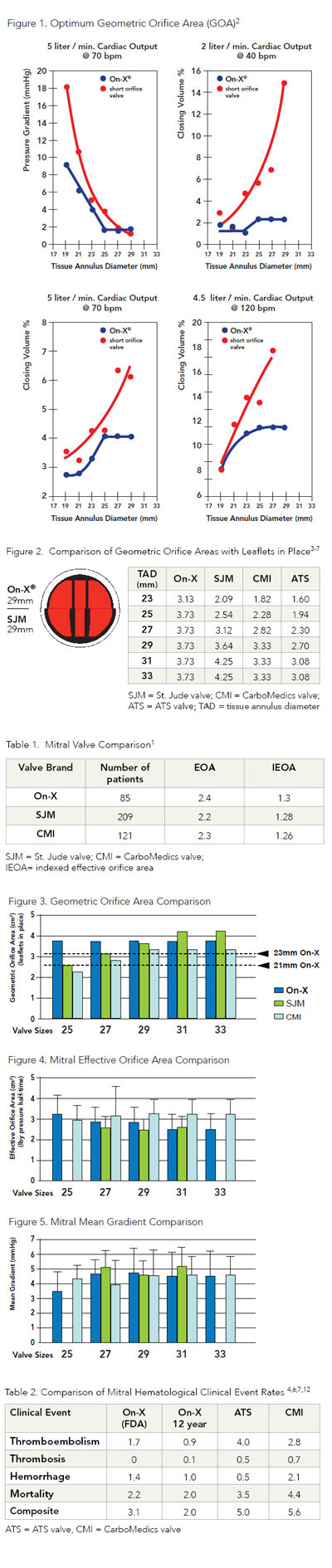
The On-X valves were designed to be less turbulent
Low complication rates, less blood destruction and low gradients provide compelling evidence that an inlet flare, near natural valve length and 90° leaflet opening angle provide less turbulence even in larger sized On-X mitral valves. Determining optimal size without gaining increased closing (or trapped) volume was taken into consideration in developing the On-X valves. As you can see in Figure 1, regurgitant closing volume increases greatly for large sizes of a short orifice valve at all heart rates—an undesirable effect.
Larger geometric orifice areas (GOA’s) with leaflets in place for the On-X valve
Sizing designations for mechanical valve orifices have not been standardized and have been confusing for the cardiovascular surgical community. A comparison of valve geometric orifice areas listed in the data provided to the United States Food and Drug Administration (US FDA) shows that only one valve manufacturer makes a larger mitral valve orifice than the On-X valve mitral and aortic orifice 25 (Figure 2).3-7
Greater effective orifice area (EOA) and less mismatch
A Canadian study shows less mismatch for the On-X mitral valve overall compared with other valves (Table 1).1 Figures 3-5 show that even though other valve brands increase the GOA for each size, the gradient and EOA values for all large sized valves are essentially the same.8-11 Therefore, increasing GOA beyond optimal flow does not make sense when increased turbulence, blood destruction and noise are a concern. Replacement of mitral regurgitation or stenosis with a prosthetic that has a large trapped volume and a limited EOA is essentially implanting regurgitant disease.
Iron man competition for an On-X mitral valve
This optimal flow has been proven to be effective even in a 6’5”, 230 pound man who completed a triathlon 10 months after his implant surgery and continues to propel him through more rigorous exercises like the half iron man competiton.
Lowest mitral complication rates for the On-X valve
In recent trials for FDA approval, the On-X valve showed the lowest overall mitral complication and mortality rates (Table 2). These low rates and reduced LDH levels are evidence of lower turbulence for the On-X valve.3-7,13
Less mismatch, lower complication and mortality rates, less closing regurgitation and low blood destruction all prove that the On-X mitral valve is the right one for your mechanical valve patients.
References
- Lorusso R, Gelsomino S, Lucá F, et al. Type II diabetes mellitus is associated with faster degeneration of bioprosthetic valve: Results from a propensity score-matched Italian multicenter study. Circulation 2011;on-line publication available at: http://circ.ahajournal.org/content/early/2011/12/27/CIRCUALTIONAHA.111.025064
- Briand M, Pibarot P, Despres JP, et al. Metabolic syndrome is associated with faster degeneration of bioprosthetic valves. Circulation 2006;114:I-512-I-517
- Nollert G, Miksch J, Kreuzer E, et al. Risk factors for atherosclerosis and the degeneration fo pericardial valves after aortic valve replacement. J Thorac Cardiovasc Surg 2003;126:965-68
- Farivar RS, Cohn LH. Hypercholesterolemia is a risk factor for bioprosthetic valve calcification and explantation. J Thorac Cardiovasc Surg 2003;126:969-75
- Flameng W, Herregods M, Vercalsteren M, et al. Prosthesis-patient mismatch predicts structural valve degeneration in bioprosthetic heart valves. Circulation 2010;121:2123-29
- Weber A, Noureddine H, Englberger L, et al. Ten-year comparison of pericardial bioprostheses and mechanical aortic valve replacement in patients less than 60 years of age. The Society of Thoracic Surgeons 47th Annual Meeting Program Book, Abstract 47, page 182
- Edwards Life Sciences Carpentier-Edwards Perimount Magna Pericardial Bioprosthesis. Instructions for Use. © 2003
- Mitroflow Aortic Pericardial Heart Valve. Summary of Safety and Effectiveness Data submitted to the United States Food and Drug Administration. PMA P060038. Approval date October 23, 2007
- SJM Biocor® Valve and SJM Biocor® Supra Valve. Summary of Safety and Effectiveness Data submitted to the United States Food and Drug Administration. PMA P040021. Approval date August 5, 2005
- Medtronic Freestyle® Aortic Root Prosthesis. Summary of Safety and Effectiveness Data submitted to the United States Food and Drug Administration. PMA P970031 Approval date November 26, 1997
- Mosaic Heart Valve. Summary of Safety and Effectiveness Data submitted to the United States Food and Drug Administration. PMA P990064. Approval date July 14, 2000
- ATS 3f® Aortic Bioprosthesis, Model 1000. Instructions for Use
- Brown ML, Schaff HV, Lahr BD, et al. Aortic valve replacement in patients aged 50 to 70 years: Improved outcome with mechanical versus biologic prostheses. J Thorac Cardiovasc Surg 2008;135:878-84
- Daneshmand MA, Milano CA, Rankin JS, et al. Influence of patient age on procedural selection in mitral valve surgery. Ann Thorac Surg 2010;90:1479-86
- Badhwar V. Ofenloch J, Rovin J, et al. Equivalency of closely monitored mechanical valves to bioprostheses overshadowed by early mortality benefit in younger patients. The Society of Thoracic Surgeons 47th Annual Meeting Program Book, Poster Abstract 12, page 359
- Vicchio M, Della Corte A, De Santo LS, et al. Tissue versus mechanical prostheses: Quality of life in octogenarians. Ann Thorac Surg 2008;85:1290-95
- de Vincentiis C, Kunkl AB, Trimarchi S, et al. Aortic valve replacement in octogenarians: Is biologic valve the unique solution? Ann Thorac Surg 2008;85:1296-302
On-X aortic and mitral valves are FDA approved. Not all On-X valve models are available in all markets.
CAUTION: Federal law restricts this device to sale by or on the order of a physician. Refer to the Instructions for Use that accompany each valve for indications, contraindications, warnings, precautions and possible complications. For further information, visit www.onxlti.com.
Headquarters and Manufacturing Facilities: 1300 East Anderson Lane, Building B Austin, Texas 78752 U.S.A.
Telephone: (512) 339-8000 – Facsimile: (512) 339-3636 – www.onxlti.com – onx@onxlti.com
010006 208 081012 © 2012 On-X Life Technologies, Inc.


