Clinical Update Thirty-Eight: Improved Mortality
Improved mortality for mechanical valve patients
Several recent clinical trials provide evidence of superior durability, reoperation rates and mortality for mechanical valve recipients compared to biological valve recipients.1-9
Matched study results favor mechanical valves
In an abstract presented earlier this year at the Society of Thoracic Surgery, Weber, et al., of Switzerland offered the following about aged-matched patients:
“In our cohort of patients less than 60 years of age, biological AVR is associated with reduced ten year survival compared to mechanical valve implantation. Despite similar valve-related event rates in both groups, better hemodynamic performance of mechanical valves determines outcomes.”1
Brown, et al., of the Mayo Clinic in a retrospective matched study similarly noted:
“We observed improved survival of patients who received mechanical prostheses. There is insufficient evidence to recommend bioprosthetic valves in the aortic position for patients younger than 65 years . . .” 2
In a propensity matched study, Badhwar, et al., observed:
“Mechanical valves begin to confer mortality benefit over bioprostheses as early as 7.5 years postoperatively.”4
In a study of mitral valves, Daneshmand and associates found:
“However, after adjustment for differences in preoperative baseline characteristics, mitral repair still had the best predicted survival, followed by mechanical valve replacement . . , and tissue valves seemed inferior to both of the other options.”3
These differences are shown in Table 1 along with other similar findings.1-9
| Study | Follow-up Period |
BP % Survival | MP % Survival | P Value | Mean Age | BP Brand | MP Brand | |
|---|---|---|---|---|---|---|---|---|
| BP | MP | |||||||
| Weber, et al.1 | 1.7 mean yrs. |
89.1 | 96.7 | p<0.05 | 51.7 | 49.7 | CE | ATS SJM |
| Brown, et al.2 | 5 year survival |
72 | 87 | p<.01 | 66.6* | 65.7* | CE | SJM |
| Daneschmand, et al.3 | 5 year survival estimated |
52 | 75 | p<0.0001 | 72* | 62* | CE | SJM |
| Badhwar, et al.4 | 5 year survival estimated |
85 | 93 | p=0.04 (7.5 yrs.) |
56.4 | 56.4 | CE Medtronic |
On-X |
| Vicchio, et al.5 | 5 year survival |
58.1 | 81.6 | p=0.025 | 82.9* | 81.8* | Not specified | Not specified |
| deVincentiis, et al.6 | 5 year survival estimated |
78 | 85 | p=0.095 | 82 | 82 | Not specified | Not specified |
| FDA submission studies7,8,9 |
5-6 year survival estimated |
73.5 (6 yrs.) |
90 (5 yrs.) |
— | 62.9 | 60.2 | CE Magna |
On-X |
Table 1. Mortality rates of tissue valves (BP) versus mechanical valves (MP)
*significant difference in age defined in study
BP = biological prosthesis, MP = mechanical prosthesis
CE = Carpentier Edwards, SJM = St. Jude Medical, ATS = Advancing the Standard (Medtronic)
Unexpected similarity in bleeding and thromboembolic rates
In a randomized eight-year study of middle-aged tissue and mechanical patients in Italy, Stassano, et al.,10 reported differences in valve failure and reoperation between the groups, but, as with Weber, et al.,1 no other major valve-related events reached significance. Additionally, 21% of the tissue valve recipients in this study needed continued warfarin therapy after surgery.
Dramatic failure rates at ten to twelve years for tissue valves
The Stassano study results mirror a similar finding from the Cleveland Clinic11 showing that the peak time for structural valve deterioration in patients less than 50 years old is between ten and twelve years after implant. This is not the 20-year life touted by some tissue valve manufacturers even though it is often erroneously the rationale given for implant of tissue valves implants in younger and younger patients.
“Valve-in-valve” in younger patients not justified
Another misleading and unproven claim is the “valve-in-valve” theory of a transcatheter valve as a possibility of a second implant after a failed tissued valve. Because of reduced survival in patients less than 60 with biological valves, Weber, et al., concluded, “The later possibility of a transcatheter valve in valve intervention does not justify the indication for biological AVR in younger patients.”1
Tissue valves produce complications equal to those of mechanical valves
Recent findings at the Mayo Clinic show unacceptable thrombosis rates for porcine tissue valve implants.12 As found in the studies cited above, complication rates for tissue valves are commonly the same as those for mechanical valves. Pannus can cause failure of tissue valve implants as well (Figure 1).
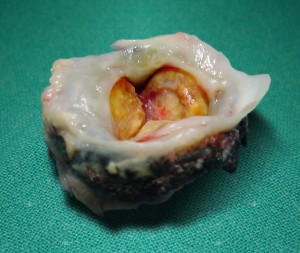
Figure 1. Pannus ingrowth causes failure of explanted tissue valve13
Matched studies and FDA approval clinical trials show that the On-X Heart Valve offers the lowest mortality and morbidity rates for your patients.1-9
References
- Weber A, Noureddine H, Englberger L, et al. Ten-year comparison of pericardial bioprostheses and mechanical aortic valve replacement in patients less than 60 years of age. The Society of Thoracic Surgeons 47th Annual Meeting Program Book, Abstract 47, page 182
- Brown ML, Schaff HV, Lahr BD, et al. Aortic valve replacement in patients aged 50 to 70 years: Improved outcome with mechanical versus biologic prostheses. J Thorac Cardiovasc Surg 2008;135:878-84
- Daneshmand MA, Milano CA, Rankin JS, et al. Influence of patient age on procedural selection in mitral valve surgery. Ann Thorac Surg 2010;90:1479-86
- Badhwar V. Ofenloch J, Rovin J, et al. Equivalency of closely monitored mechanical valves to bioprostheses overshadowed by early mortality benefit in younger patients. The Society of Thoracic Surgeons 47th Annual Meeting Program Book, Poster Abstract 12, page 359
- Vicchio M, Della Corte A, De Santo LS, et al. Tissue versus mechanical prostheses: Quality of life in octogenarians. Ann Thorac Surg 2008;85:1290-95
- de Vincentiis C, Kunkl AB, Trimarchi S, et al. Aortic valve replacement in octogenarians: Is biologic valve the unique solution? Ann Thorac Surg 2008;85:1296-302
- On-X® Prosthetic Heart Valve. Summary of Safety and Effectiveness Data submitted to the United States Food and Drug Administration. PMA P000037. Approval date May 30, 2001 and October 11, 2002
- Edwards Life Sciences Carpentier-Edwards Perimount Magna Pericardial Bioprosthesis. Instructions for Use. © 2003
- Palatianos GM, Laczkovics AM, Simon P, et al. Multicentered European study on safety and effectiveness of the On-X Prosthetic Heart Valve: Intermediate follow-up. Ann Thorac Surg 2007;83:40-46
- Stassano P, Di Tommaso L, Monaco M, et al. Aortic valve replacement: A prospective randomized evaluation of mechanical versus biological valves in patients ages 55 to 70 years. J Am Coll Cardiol 2009;54:1862–68
- Smedira NG, Blackstone EH, Roselli EE, et al. Are allografts the biologic valve of choice for aortic valve replacement in nonelderly patients? Comparison of explantation for structural valve deterioration of allograft and pericardial prostheses. J Thorac Cardiovasc Surg 2006;131:558-64
- Brown ML, Park SJ, Sundt TM, Schaff HV. Early thrombosis risk in patients with biologic valves in the aortic position. Article in press. © 2011 The American Association for Thoracic Surgery, Published by Elsevier Inc.
- Photo courtesy of On-X Life Technologies, Inc., Austin, TX, USA 2011
On-X aortic and mitral valves are FDA approved.
CAUTION: Federal law restricts this device to sale by or on the order of a physician. Refer to the Instructions for Use that accompany each valve for indications, contraindications, warnings, precautions and possible complications. For further information, visit www.onxlti.com.
Headquarters and Manufacturing Facilities: 1300 East Anderson Lane, Building B Austin, Texas 78752 U.S.A.
Telephone: (512) 339-8000 – Facsimile: (512) 339-3636 – www.onxlti.com – onx@onxlti.com
010006 201 121611 © 2011 On-X Life Technologies, Inc.


