Clinical Update Thirty-Three: On-X Pure Carbon Valve
Does the On-X Pure Carbon Valve behave like a mechanical or tissue valve?
Results of an intermediate term study of 737 Canadian patients show decreased risk with the On-X® Prosthetic Heart Valve.1
A study published in the November 2010 issue of The Journal of Thoracic and Cardiovascular Surgery found approximately 30% improvement in aortic valve morbid event rates for the On-X valve and approximately 50% improvement in mitral valve morbid even rates compared with other mechanical valves’ rates. The most stunning difference is the low level of hemorrhagic events for the On-X valve compared with other mechanical valve studies—an indication that On-X patients were being maintained at very moderate levels of anticoagulation. The low level of anticoagulation and reduced hemorrhagic events for On-X patients did not lead to increased thromboembolic (TE) events. These findings for On-X patients emulate those for tissue valves.
The authors of this paper from two prestigious academic Canadian centers studied 737 patients implanted with the On-X valve from 2003 to 2008. Average intermediate follow-up for the study was 2.8 years. Their findings confirm the previous trend for the On-X valve to produce the lowest complication rates seen to date for mechanical valve technology.
The comparison in this publication reflects a similar advantageous finding for the On-X valve in studies conducted for mechanical valves for FDA market approval in the USA. Comparison to tissue valve studies conducted for the FDA further reveals the similarity to On-X valve results (Tables 1 and 2).
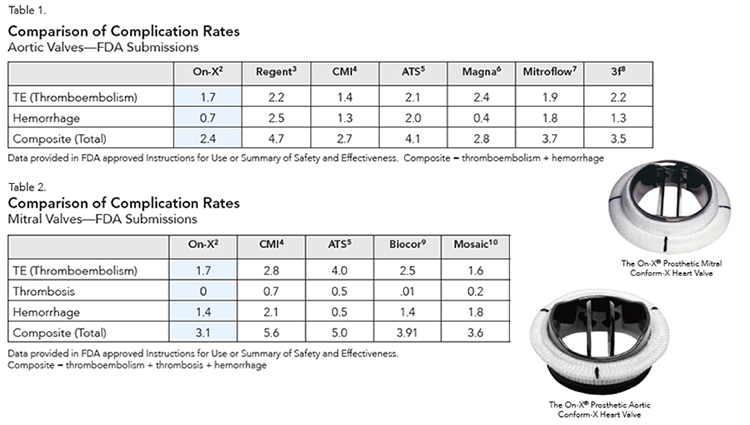
Studies conducted under protocols approved by the FDA for market approval in the United States are closely monitored, international multi-center studies that yield the best possible comparison of clinical values next to randomized trials because:
- Randomized trials which are theoretically better are difficult to conduct with different prostheses due to unwillingness of participants to randomize in lieu of surgeon choice/patient condition and/or patient choice.
- Single center comparisons can fall short of objectivity since patients may be selected to give the “best results”.
When a “mechanical valve” is indicated, choose the valve “designed for life” – the On-X® Pure Carbon Valve!
References
- Chan V, Jamieson WRE, Lam BK, et al. Influence of the On-X mechanical prosthesis on intermediate-term major thromboembolism and hemorrhage: A prospective multicenter study. J Thorac Cardiovasc Surg 2010;140:1053-58
- On-X On-X® Prosthetic Heart Valve. Summary of Safety and Effectiveness Data submitted to the United States Food and Drug Administration. PMA P000037. Approval date May 30, 2001 and October 11,2002
- SJM Regent® Valve. Clinical Study Summary (package insert)
- CarboMedics® Prosthetic Heart Valve. Summary of Safety and Effectiveness Data submitted to the United States Food and Drug Administration. PMA P900060. Approval date April 13, 1993
- ATS Open Pivot® Bileaflet Heart Valve. Summary of Safety and Effectiveness Data submitted to the United States Food and Drug Administration. PMA P990046. Approval date October 13, 2000
- Edwards Life Sciences Carpentier-Edwards Perimount Magna Pericardial Bioprosthesis. Instructions for Use. Copyright 2003
- Mitroflow Aortic Pericardial Heart Valve. Summary of Safety and Effectiveness Data submitted to the United States Food and Drug Administration. PMA P060038. Approval date October 23, 2007
- ATS 3f® Aortic Bioprosthesis, Model 1000. Instructions for Use
- SJM Biocor® Valve and SJM Biocor® Supra Valve. Summary of Safety and Effectiveness Data submitted to the United States Food and Drug Administration. PMA P040021. Approval date August 5, 2005
- Mosaic Heart Valve. Summary of Safety and Effectiveness Data submitted to the United States Food and Drug Administration. PMA P990064. Approval date July 14, 2000.
On-X aortic and mitral valves are FDA approved. CAUTION: Federal law restricts this device to sale by or on the order of a physician. Refer to the Instructions for Use that accompany each valve for indications, contraindications, warnings, precautions and possible complications. For further information, visit www.onxlti.com.
Headquarters and Manufacturing Facilities: 1300 East Anderson Lane, Building B Austin, Texas 78752 U.S.A.
Telephone: (512) 339-8000 – Facsimile: (512) 339-3636 – www.onxlti.com – onx@onxlti.com
010006 180 121010 © 2010 On-X Life Technologies, Inc.


